
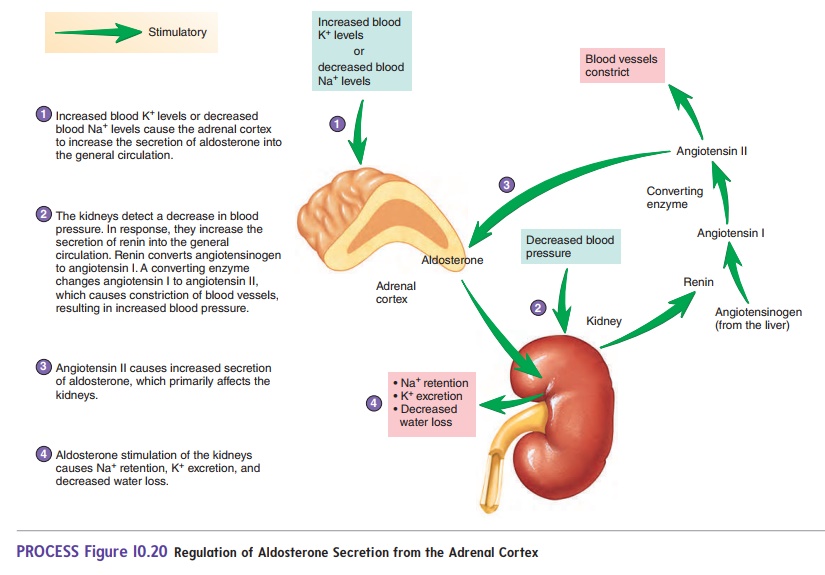
Overall, we show three major findings: ( i) APCCs are common in normal adrenals, ( ii) APCCs harbor somatic mutations known to cause excess aldosterone production, and ( iii) the mutation spectrum of aldosterone-driving mutations is different in APCCs from that seen in APA. Known aldosterone driver mutations were identified in 8 of 23 (35%) APCCs, including mutations in calcium channel, voltage-dependent, L-type, α1D-subunit ( CACNA1D 6 of 23 APCCs) and ATPase, Na + /K + transporting, α1-polypeptide ( ATP1A1 2 of 23 APCCs), which were not observed in the adjacent normal adrenal tissue. To determine if APCCs harbored APA-related mutations, we performed targeted next generation sequencing of DNA from 23 APCCs and adjacent normal adrenal tissue isolated from both formalin-fixed, paraffin-embedded, and frozen tissues. The APCC transcriptome was most similar to ZG but with an enhanced capacity to produce aldosterone. To clarify APCC molecular characteristics, we used microarrays to compare the APCC transcriptome with conventional adrenocortical zones. APCCs were studied in 42 normal adrenals from kidney donors. Herein, we hypothesized that APCCs have APA-related aldosterone-stimulating somatic gene mutations.
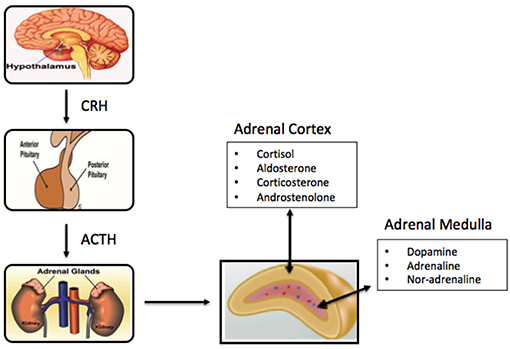
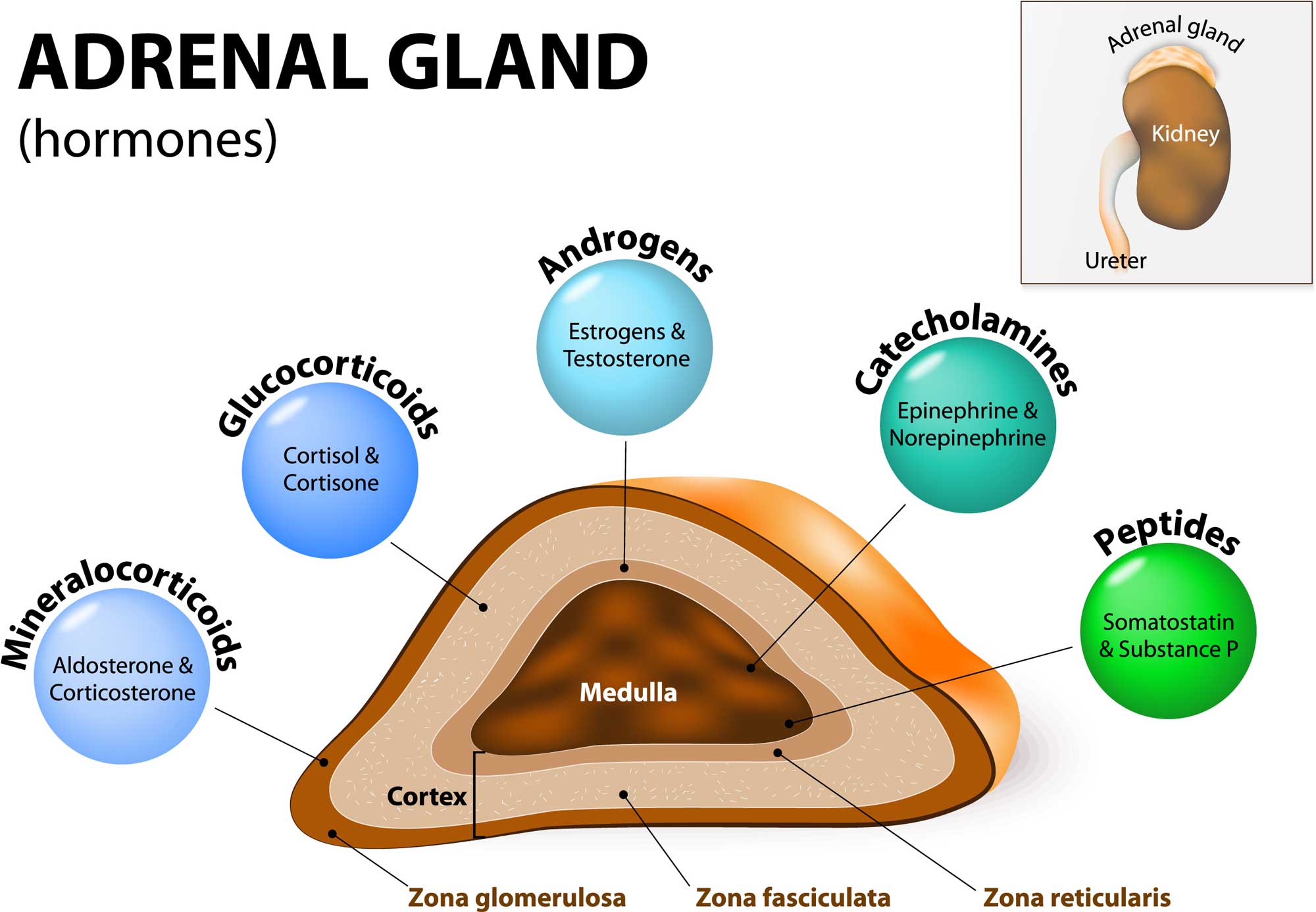
PA-causing aldosterone-producing adenomas (APAs) harbor mutations in genes encoding ion channels/pumps that alter intracellular calcium homeostasis and cause renin-independent aldosterone production through increased CYP11B2 expression. Recently, aldosterone-producing cell clusters (APCCs) with high expression of aldosterone synthase (CYP11B2) were found in both normal and PA adrenal tissue. Primary aldosteronism (PA) represents the most common cause of secondary hypertension, but little is known regarding its adrenal cellular origins.


 0 kommentar(er)
0 kommentar(er)
